

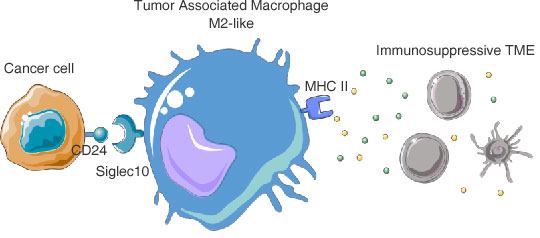
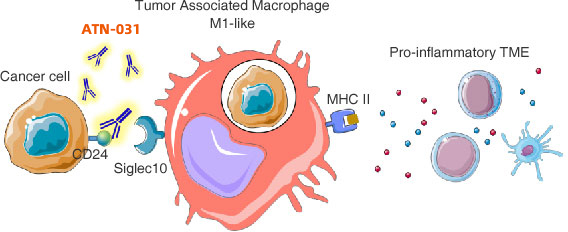
Primary objectives:Safety, tolerability. Define MTD and RP2D
Secondary objectives:Evaluate preliminary efficacy and pharmacology
RP2D dose evaluation as monotherapy or combo with chemotherapy or immunotherapy
ADA: anti-drug antibody: MTD = maximally tolerated dose, RP2D = recommended phase 2 dose
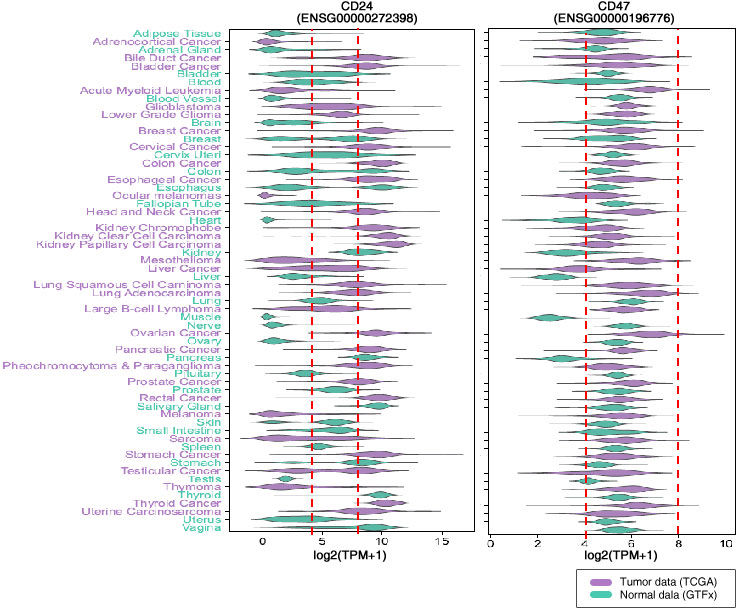
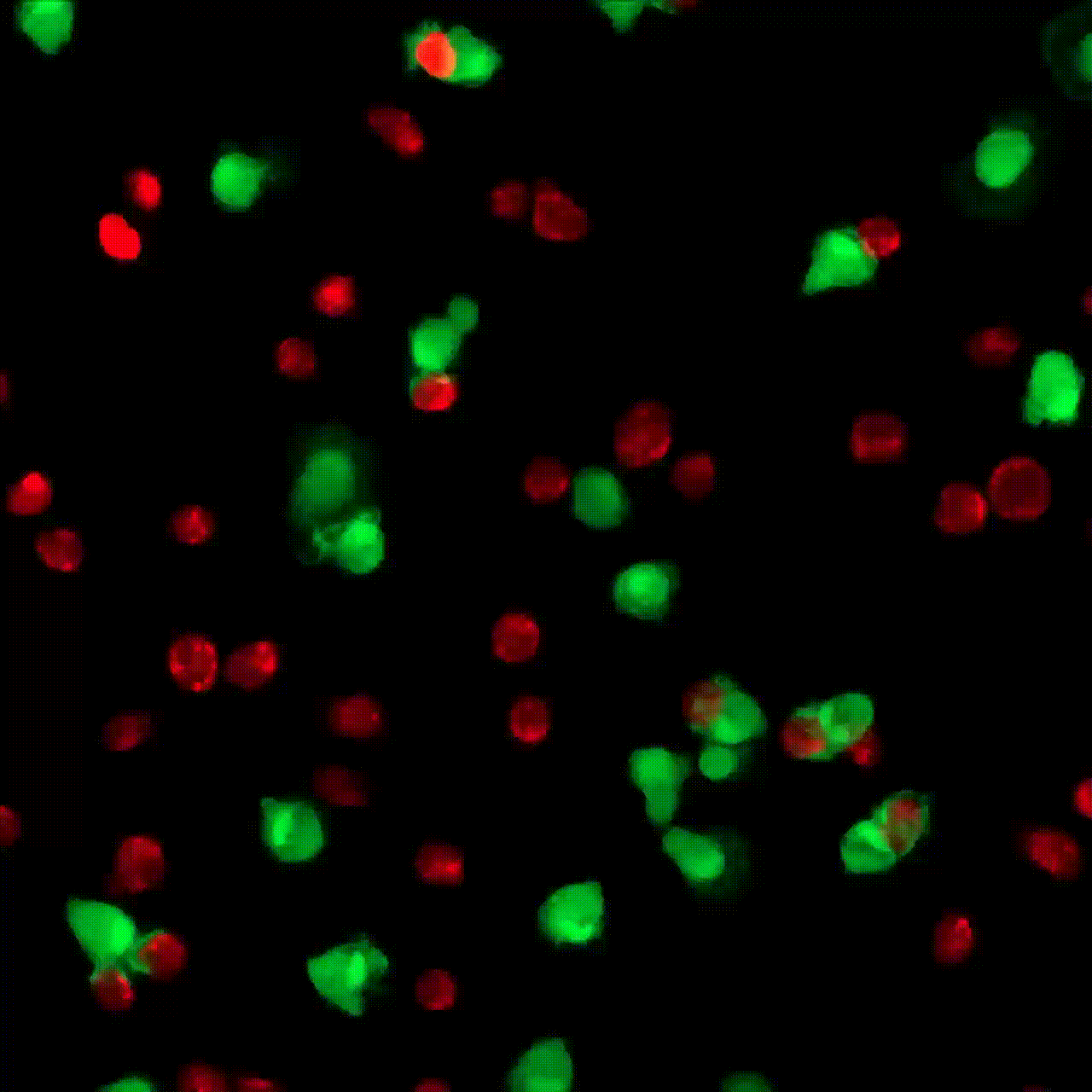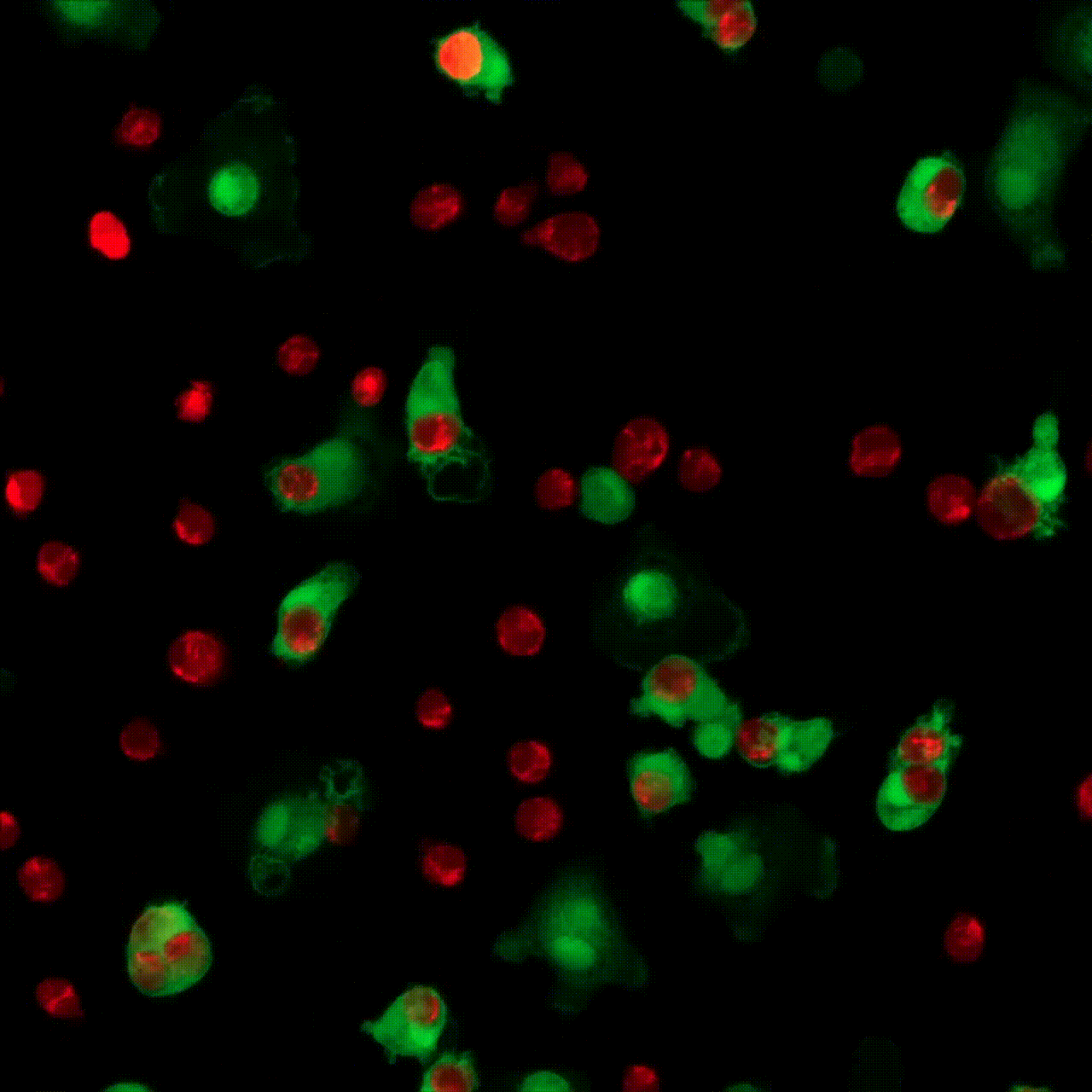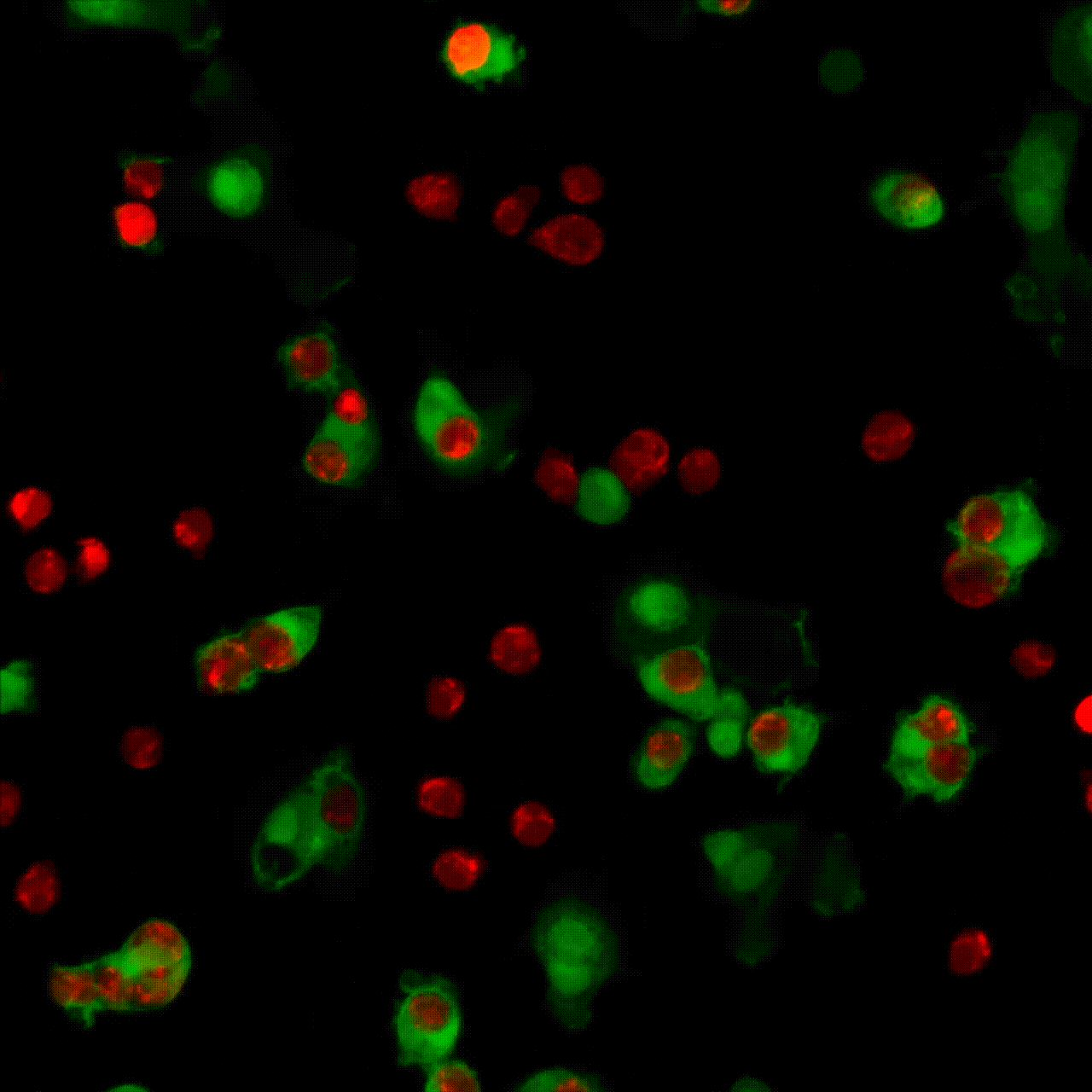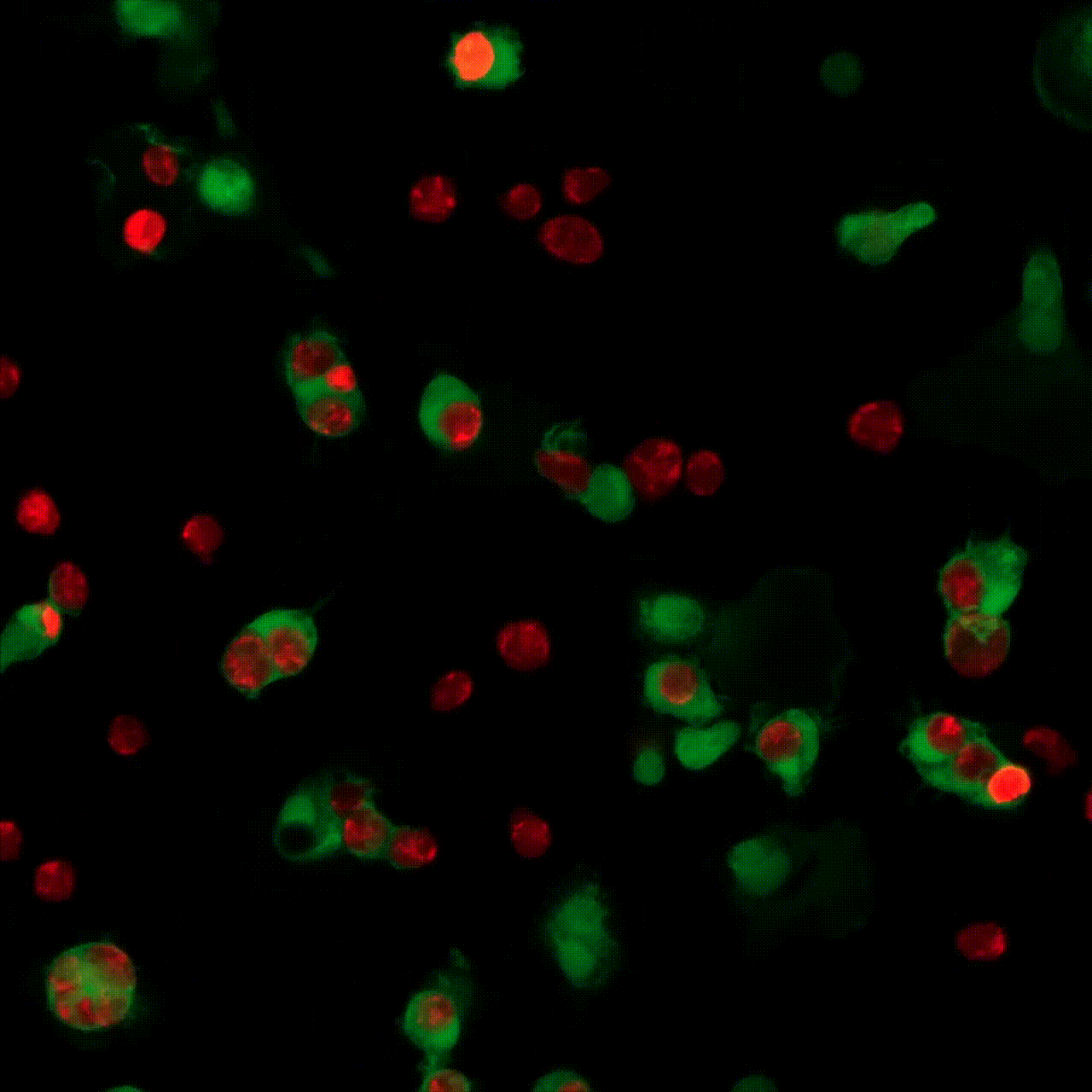
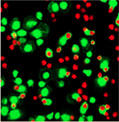
5 minutes
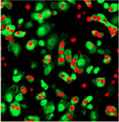
30 minutes
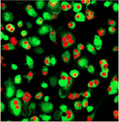
1 hour
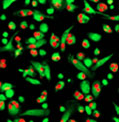
5 hours
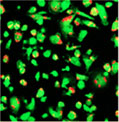
10 hours
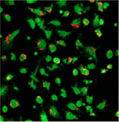
20 hours
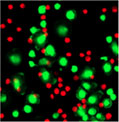
5 minutes
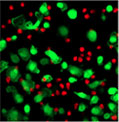
30 minutes
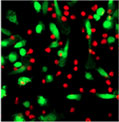
1 hour
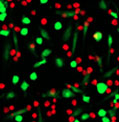
5 hours
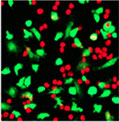
10 hours
20 hours
